|
|
vor 3 Jahren | |
|---|---|---|
| R | vor 3 Jahren | |
| img | vor 3 Jahren | |
| inst | vor 3 Jahren | |
| man | vor 3 Jahren | |
| src | vor 3 Jahren | |
| .DS_Store | vor 3 Jahren | |
| .gitattributes | vor 3 Jahren | |
| .gitignore | vor 3 Jahren | |
| DESCRIPTION | vor 3 Jahren | |
| LICENSE | vor 3 Jahren | |
| NAMESPACE | vor 3 Jahren | |
| README.md | vor 3 Jahren |
README.md
CALDER user manuel
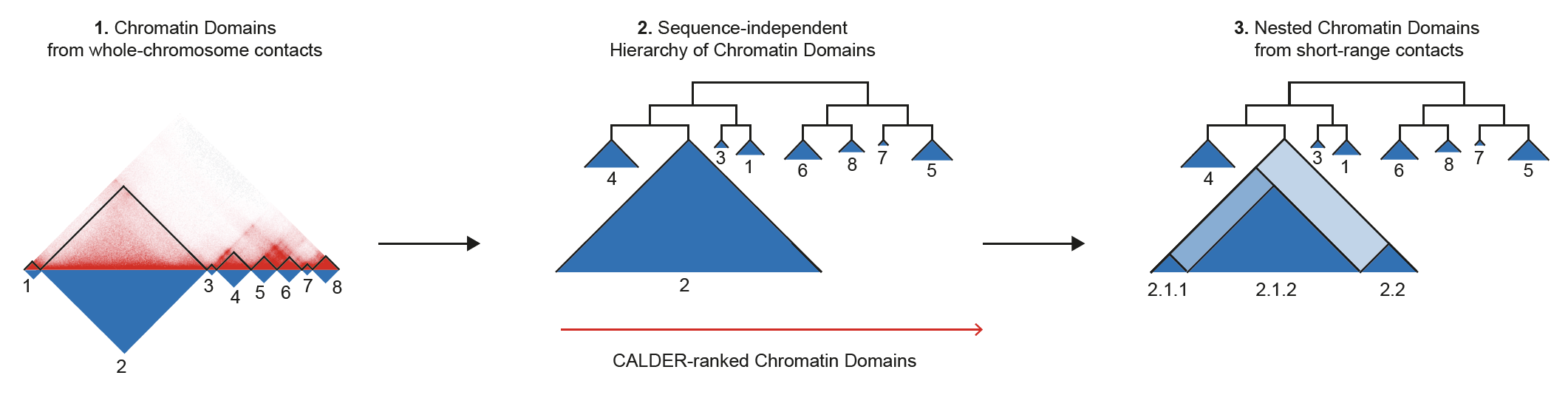
CALDER is a Hi-C analysis tool that allows: (1) compute chromatin domains from whole chromosome contacts; (2) derive their non-linear hierarchical organization and obtain sub-compartments; (3) compute nested sub-domains within each chromatin domain from short-range contacts. CALDER is currently implemented in R.
Multiple new features were added in version 2.0
- Support for hg19, hg38, mm9, mm10 and other genomes
- Support input in .hic format generated by Juicer tools (https://github.com/aidenlab/juicer)
- Opitimized bin_size selection
- Aggregated all chromosome output into a single file
- Added output in tabular .txt format at bin level for downstream analysis
Introduction of opitimized bin_size selection
Due to reasons such as low data quality or large scale structrual variation, compartments can be unrealiablly called at one bin_size (equivalent to resoltution in the literature) but might be captured at another bin_size. We added an opitimized bin_size selection strategy to call reliable compartments. It is based on the observation from our large scale compartment analysis (https://www.nature.com/articles/s41467-021-22666-3) that, although compartments can change between different conditions, their overall correlation cor(compartment_rank_1, compartment_rank_2) is high (> 0.4).
Given a bin_size specified by user, we call compartments with extended bin_sizes and choose the smallest bin_size such that no bigger bin_size can increase the correclation with a reference compartment more than 0.05. For example, if correclation for bin_size=10000 is 0.2 while for bin_size=50000 is 0.6, we are more confident the latter is more reliable; if correclation for bin_size=10000 is 0.5 while for bin_size=50000 is 0.52, we would choose the former as it has higher resolution.
bin_size is extended in the following way such that we can aggregated directly from the input contact matrix into larger bin_sizes
if(bin_size==5E3) bin_sizes = c(5E3, 10E3, 50E3, 100E3)
if(bin_size==10E3) bin_sizes = c(10E3, 50E3, 100E3)
if(bin_size==20E3) bin_sizes = c(20E3, 40E3, 100E3)
if(bin_size==25E3) bin_sizes = c(25E3, 50E3, 100E3)
if(bin_size==40E3) bin_sizes = c(40E3, 80E3)
if(bin_size==50E3) bin_sizes = c(50E3, 100E3)
Note taht this strategy is currently only available for the hg19, hg38, mm9, mm10 genome for which we generated high quality reference compartments, using Hi-C data: from GSE63525 for hg19, from https://data.4dnucleome.org/files-processed/4DNFI1UEG1HD/ for hg38, from GSM3959427 for mm9, from http://hicfiles.s3.amazonaws.com/external/bonev/CN_mapq30.hic for mm10.
Installation
Make sure all dependencies have been installed:
- R.utils (>= 2.9.0),
- doParallel (>= 1.0.15),
- ape (>= 5.3),
- dendextend (>= 1.12.0),
- fitdistrplus (>= 1.0.14),
- igraph (>= 1.2.4.1),
- Matrix (>= 1.2.17),
- rARPACK (>= 0.11.0),
- factoextra (>= 1.0.5),
- maptools (>= 0.9.5),
- data.table (>= 1.12.2),
- fields (>= 9.8.3),
- GenomicRanges (>= 1.36.0)
- ggplot2 (>= 3.3.5)
- strawr (>= 0.0.9)
Clone its repository and install it from source:
git clone https://github.com/CSOgroup/CALDER.git
install.packages(path_to_CALDER, repos = NULL, type="source") ## install from the cloned source file
Please contact yliueagle@googlemail.com for any questions about installation.
install CALDER and dependencies automaticly:
if (!requireNamespace("BiocManager", quietly = TRUE))
install.packages("BiocManager")
BiocManager::install("GenomicRanges")
install.packages("remotes")
remotes::install_github("CSOgroup/CALDER")
Usage
The input data of CALDER is a three-column text file storing the contact table of a full chromosome (zipped format is acceptable, as long as it can be read by data.table::fread). Each row represents a contact record pos_x, pos_y, contact_value, which is the same format as that generated by the dump command of juicer (https://github.com/aidenlab/juicer/wiki/Data-Extraction):
16050000 16050000 10106.306
16050000 16060000 2259.247
16060000 16060000 7748.551
16050000 16070000 1251.3663
16060000 16070000 4456.1245
16070000 16070000 4211.7393
16050000 16080000 522.0705
16060000 16080000 983.1761
16070000 16080000 1996.749
...
A demo dataset is included in the repository CALDER/inst/extdata/mat_chr22_10kb_ob.txt.gz and can be accessed by system.file("extdata", "mat_chr22_10kb_ob.txt.gz", package='CALDER') once CALDER is installed. This data contains contact values of GM12878 on chr22 binned at 10kb (Rao et al. 2014)
CALDER contains three modules: (1) compute chromatin domains; (2) derive their hierarchical organization and obtain sub-compartments; (3) compute nested sub-domains within each compartment domain.
To run three modules in a single step:
CALDER_main(contact_mat_file,
chr,
bin_size,
out_dir,
sub_domains=TRUE,
save_intermediate_data=FALSE,
genome='hg19')
To run three modules in seperated steps:
# This will not compute sub-domains, but save the intermediate_data that can be used to compute sub-domains latter on
CALDER_main(contact_mat_file,
chr,
bin_size,
out_dir,
sub_domains=FALSE,
save_intermediate_data=TRUE,
genome='hg19')
# (optional depends on needs) Compute sub-domains using intermediate_data_file that was previous saved in the out_dir (named as chrxx_intermediate_data.Rds)
CALDER_sub_domains(intermediate_data_file,
chr,
out_dir,
bin_size)
Paramters:
contact_mat_file: path to the contact table of a chromosomechr: chromosome number. Either numeric or character, will be pasted to the output namebin_size: numeric, the size of a bin in consistent with the contact tableout_dir: the output directorysub_domains: logical, whether to compute nested sub-domainssave_intermediate_data: logical. If TRUE, an intermediate_data will be saved. This file can be used for computing nested sub-domains later ongenome: string. Specifies the genome assembly (Default="hg19").
| Parameters | Description |
| --------------------- | ----------------------- |
| chrs | A vector of chromosome names to be analyzed, with or without 'chr'
| contact_file_dump |A list of contact files in dump format, named by chrs. Each contact file stores the contact information of the corresponding chr. Only one of contact_file_dump, contact_tab_dump, contact_file_hic should be provided
| contact_tab_dump | A list of contact table in dump format, named by chrs, stored as an R object. Only one of contact_file_dump, contact_tab_dump, contact_file_hic should be provided
| contact_file_hic | A hic file generated by Juicer tools. It should contain all chromosomes in chrs. Only one of contact_file_dump, contact_tab_dump, contact_file_hic should be provided
| ref_genome | One of 'hg19', 'hg38', 'mm9', 'mm10', 'others' (default). These compartments will be used as reference compartments for optimized bin_size selection. If ref_genome = others, an annotation_track should be provided (see below) and no optimized bin_size selection will be performed
| annotation_track | A genomic annotation track in data.frame or data.table format. This track will be used for determing the A/B compartment direction when ref_genome=others and should presumably have higher values in A than in B compartment. Some suggested tracks can be gene density, H3K27ac, H3K4me1, H3K4me2, H3K4me3, H3K36me3 (or negative transform of H3K9me3 signals)
| bin_size | The bin_size (resolution) to run CALDER. bin_size should be consistent with the data resolution in contact_file_dump or contact_tab_dump if these files are provided as input, otherwise bin_size should exist in the contact_file_hic file. Recommended bin_size is between 10000 to 50000
| save_dir | the directory to save outputs
| save_intermediate_data | logical. If TRUE, an intermediate_data will be saved. This file can be used for computing nested sub-domains later on
| n_cores | integer. Number of cores to be registered for running CALDER in parallel
| single_binsize_only | logical. If TRUE, CALDER will compute compartments only using the bin_size specified by the user and not do bin size optimization
| sub_domains | logical, whether to compute nested sub-domains
Output:
chrxx_domain_hierachy.tsv
- information of compartment domain and their hierarchical organization. The hierarchical structure is fully represented by
compartment_label, for example,B.2.2.2andB.2.2.1are two sub-branches ofB.2.2. Thepos_endcolumn specifies all compartment domain borders, except when it is marked asgap, which indicates it is the border of a gap chromsome region that has too few contacts and was excluded from the analysis (e.g., due to low mappability, deletion, technique flaw)
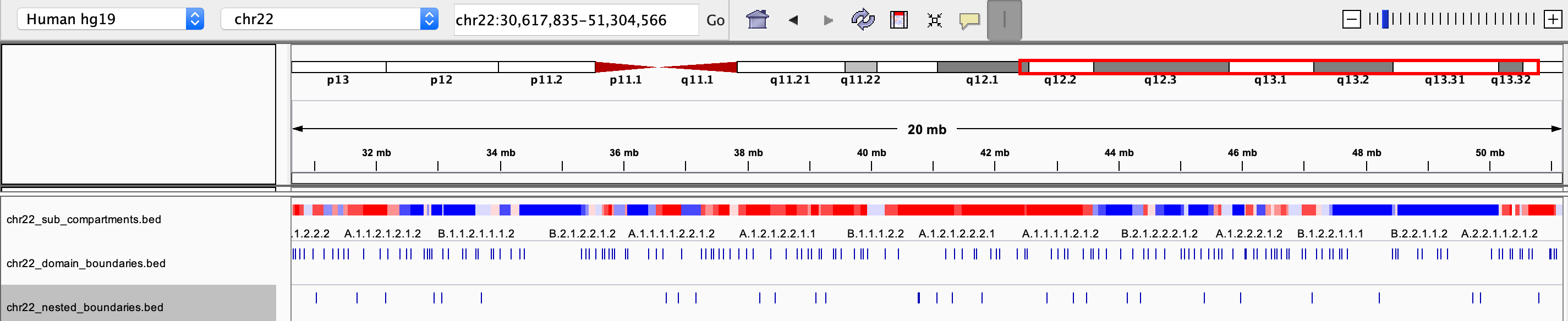
chrxx_sub_compartments.bed
- a .bed file containing the sub-compartment information, that can be visualized in IGV. Different colors were used to distinguish compartments (at the resolution of 8 sub-compartments)
chrxx_domain_boundaries.bed
- a .bed file containing the chromatin domains boundaries, that can be visualized in IGV
chrxx_nested_boundaries.bed
- a .bed file containing the nested sub-domain boundaries, that can be visualized in IGV
chrxx_intermediate_data.Rds
- an Rds file storing the intermediate_data that can be used to compute nested sub-domains (if CALDER is run in two seperated steps)
chrxx_log.txt, chrxx_sub_domains_log.txt
- log file storing the status and running time of each step
Output Structure
The output of the workflow is stored in the folder specified by --save_dir ("results" by default) and will look like this:
results/
└── HiC_sample_1
├── 100000
│ └── KR
│ ├── chr1
│ │ ├── chr1_domain_boundaries.bed
│ │ ├── chr1_domain_hierachy.tsv
│ │ ├── chr1_log.txt
│ │ ├── chr1_nested_boundaries.bed
│ │ ├── chr1_sub_compartments.bed
│ │ └── chr1_sub_domains_log.txt
Runnig time:
For the computational requirement, running CALDER on the GM12878 Hi-C dataset at bin size of 40kb took 36 minutes to derive the chromatin domains and their hierarchy for all chromosomes (i.e., CALDER Step1 and Step2); 13 minutes to derive the nested sub-domains (i.e., CALDER Step3). At the bin size of 10kb, it took 1 h 44 minutes and 55 minutes correspondingly (server information: 40 cores, 64GB Ram, Intel(R) Xeon(R) Silver 4210 CPU @ 2.20GHz). The evaluation was done using a single core although CALDER can be run in a parallel manner.
Demo run:
library(CALDER)
contact_mat_file = system.file("extdata", "mat_chr22_10kb_ob.txt.gz", package = 'CALDER')
CALDER_main(contact_mat_file, chr=22, bin_size=10E3, out_dir='./GM12878', sub_domains=TRUE, save_intermediate_data=FALSE)
The saved .bed files can be view directly through IGV:
Citation
If you use CALDER in your work, please cite: https://www.nature.com/articles/s41467-021-22666-3
Contact information
- Author: Yuanlong LIU
- Affiliation: Computational Systems Oncology group, Department of Computational Biology, University of Lausanne, Switzerland
- Email: yliueagle@googlemail.com